Five days into India’s mass vaccination program me, there are some concerns over vaccine hesitancy. At this crucial moment in the fight against the novel coronavirus pandemic, two of the country’s most eminent vaccine scientists weigh in on some old and new questions.
Vaccines are a product of science. If the scientific method and process is followed scrupulously, based on data and evidence, there should be little room for doubt. This is a good time to revisit some of the usual questions regarding vaccines, and to address fresh questions that are emerging.
How do vaccines work; and do they help?
A vaccine is a substance that resembles the disease-causing agent (called pathogen, the coronavirus in this case) that trains the body’s immune system and creates a ‘memory’. When the pathogen infects in future, this memory is rapidly deployed to destroy it and prevent disease.
Evidence shows that incidence of an infectious disease goes down rapidly following the deployment of vaccines against it; several human diseases are now vaccine preventable. Besides the eradication of smallpox and near eradication of polio, vaccines have resulted in the prevention of over 20 other life-threatening diseases, avoiding an estimated 2-3 million deaths annually. India’s Universal Immunization Program me, among the world’s largest, immunizes about 26 million children every year. It is estimated that every dollar spent on childhood vaccines adds $44 to the economy by ensuring that children grow up to be healthy adults.
THE EXPERTS Shahid Jameel, one of India’s best known virologists, is currently director of Trivedi School of Biosciences as Ashoka University. He has previously worked with the Delhi-based International Centre for Genetic Engineering and Biotechnology (ICGEB) and served as chief executive of the Welcome Trust/DBT Alliance which funds health research. Virander Singh Chauhan is a former director of ICGEB. He is best known for his efforts towards developing a vaccine for malaria.
Vaccines take long to develop. How could the Covid-19 vaccines be ready so soon?
It can indeed take several years to develop a vaccine. After a proof of concept has been established in research laboratories, controlled manufacturing processes are developed to make stable and highly pure products that are tested on animals and then on humans for safety and effectiveness. Clinical trials in human beings are carried out in three phases to seek specific answers.
Phase 1: is done in typically 20-100 healthy volunteers to see if the vaccine is safe, if it appears to work, whether there are any serious side effects, and if these are related to the size of the dose.
Phase 2: uses several hundred volunteers to determine the most common short-term side effects, and how well the immune system responds to the vaccine — what is known as ‘immunogenicity’.
Phase 3: involves thousands of volunteers in a blinded manner to compare those who get the vaccine to those who don’t (they get a placebo or dummy) to re-confirm safety, serious side-effects if any, and most importantly, whether the vaccine is efficacious in preventing infection and/or disease.
In the present case, vaccines for Covid-19 have been readied within a year. There are currently 68 Covid-19 vaccines in human clinical trials, of which 20 have reached phase 3 testing, eight have received limited or emergency use approval, and two have been approved for full use.
There are several reasons why Covid-19 vaccines were developed so quickly. Scientific information has been shared openly, and it helped that the virus was similar to the SARS-CoV-1 and MERS viruses on which considerable work had already been done. It took just 63 days from the availability of the genome sequence (on January 11, 2020), for the Moderna mRNA-1273 vaccine to enter phase 1 trial in the United States.
Regulators have also allowed parallel phases of clinical testing and reviewing of data to expedite the process. Large investments by governments and innovative financial models allowed pharmaceutical companies to work on developing the vaccine without having to absorb all the financial risk.
Another crucial reason was the use of every available vaccine platform to produce a Covid-19 vaccine, including those that had so far not produced a vaccine for humans. The vaccines made by Pfizer/Biotech and Modena both directly deliver an mRNA fragment into human cells to produce the viral Spike protein which raises anti-viral immunity. This technology had been in development for about a decade for anti-cancer vaccines.
Similarly, non-replicating viral vectors had been in development for years. An experimental adenovirus-based Ebola vaccine was used to vaccinate about 60,000 people in West Africa during the 2014-16 Ebola outbreak. Researchers at Oxford University in the United Kingdom had been using the chimpanzee adenovirus platform for several experimental vaccines, which was quickly repurposed to develop a Covid-19 vaccine. Finally, vaccines based on inactivated viruses is a time-tested method, which has been used in the ICMR/Bharat Biotech vaccine as also in at least three vaccines from China.
It is important to also understand the limitations of each platform. The mRNA is a fragile molecule that requires protection, including frozen storage, which complicates its rollout logistics. Viral vector vaccines are more stable (2 to 8 deg C storage), but the same vector cannot be used for another disease in the same person because anti-vector immunity will make it ineffective. Though inactivated viral vaccines are generally safe, similar vaccines against respiratory syncytial virus and measles were withdrawn as they exacerbated the disease.
Did the Covid-19 vaccines get approval prematurely?
The pandemic has presented a unique opportunity to compress the vaccine development timeline without compromising on safety. Regulators have invoked Emergency Use Authorization (EUA), which is a mechanism to facilitate the availability of vaccines during public health emergencies. EUA does not compromise on safety, and includes a review of all phase 1 and phase 2 data and up to two months (for US FDA) or 70 days (for European Medicines Agency) of phase 3 follow-up, including an interim analysis for efficacy. It allows vaccines to be used in an emergency in groups that are at high risk of infection, morbidity, and mortality.
In India, the approval to Bharat Biotech’s Covian in “clinical trial mode” did lead to some confusion. It was also described as a “backup” vaccine, which could have suggested to some that it was somehow inferior to the other vaccine. As we have already noted above, Covian is based on a time-tested technology, which most likely makes it very safe.
EUA to drugs and vaccines are legitimate methods to deal with a medical emergency, but the way in which this issue was communicated in India left much to be desired.
Will the vaccines work against variant viruses?
Though coronaviruses mutate slower than other RNA viruses, new variants have emerged independently in the UK, South Africa, and Brazil that have now spread to over 50 countries, including India. These viruses have key changes in the Spike protein, allowing them to better attach to and enter cells. They multiply and transmit more efficiently, estimated at 30% to 70% more efficiently for the UK variant.
A key mutation called N501Y is found in the receptor-binding domain of the Spike protein, which is also the target of virus-neutralizing antibodies. While work is on in multiple laboratories to directly test this, some early data show that viruses with or without this mutation are neutralized equally well by the blood serum of recovered Covid-19 patients.
Variant viruses don’t always come from foreign shores. They can also emerge within. Increased genomic surveillance of infected persons within the country will provide early warning of this. However, our sequencing density is very low, with only about 5,000 virus sequences available from over 10 million confirmed cases in India. This has to increase, especially now that vaccines are being deployed, which would put additional pressure on viruses to mutate.
Any cases of vaccine failure — such as those who get the disease even after getting fully vaccinated — should be investigated for what viral variants they harbor, and if these can be neutralized by the sera of recovered patients and vaccinated persons.
It is advisable to take the vaccine because we do not fully understand the duration of protection following a natural infection. Available data suggest that neutralizing antibodies wane off in 3 to 5 months, but other arms of the immune response are likely to protect longer. If vaccine supplies are limited, which is unlikely in India, people with prior infection may delay their vaccination by a few months.


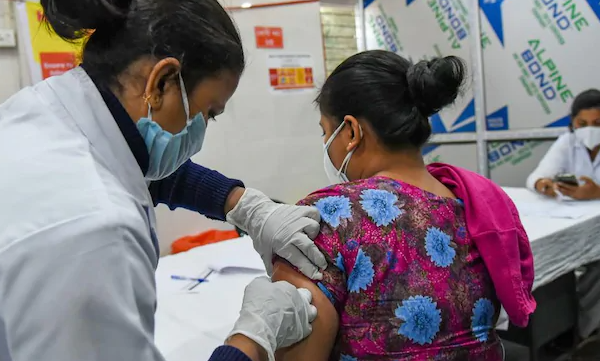

It has been five days since India began vaccination against the novel coronavirus disease. More than 7 lakh people have been given one of the two vaccines approved by the regulator. But several people, including some doctors and other healthcare workers, continue to be hesitant. This has led to concerns over ‘vaccine hesitancy’.